Executive Department for
Press, Communication and Marketing
Adolf-Reichwein-Straße 2a Gebäude AVZ (Gebäudeteil AR-NA) 57068 Siegen
Phone: +49 (0)271/740-4915 Fax.: +49 (0)271/740-4911 E-Mail: presse@uni-siegen.de
Computer-Based Simulations of Metastatic Formation
Researchers at the Universities of Siegen and Gießen are presenting a new computer model for simulating how cells, which have previously separated from a primary tumor and traveled through the vascular system, penetrate into healthy tissue. Their insights can potentially improve the prediction of effectiveness of drug-based tumor therapies.
Cancer is responsible for almost ten million deaths annually worldwide. The primary cause of cancer-related death is the formation of metastases, an aftereffect of the tumor itself. While this phenomenon is one of the primary characteristics of cancer, it has not been explored in full to date. Given that, the probability of successful cancer treatment sinks significantly once metastases have begun forming, tools for predicting tumor growth and metastatic formation would be very useful. In this field computer simulations can deliver crucial contributions. And this is where researcher from the Universities of Siegen and Gießen are looking to help.
Senior Lecturer Dr. Stephan Bäurle, head of the Theoretical Chemistry group at the University of Siegen, together with his doctoral student Kay M. Schneider and Cellular Biologist Prof. Klaudia Giehl (Justus-Liebig-Universität Gießen), developed a new method for computer-aided simulation of metastatic formation. “We can incorporate the interplay between various mechanical, chemical, and biological factors,” explains Dr. Bäurle. “The simulations contribute to understanding the mechanisms of metastasis and ultimately to predictions of the effectiveness and risks associated with drug-based tumor therapies.” The newly developed computer algorithm is capable of simulating the complete process.
However, before a simulation could be carried out, the researchers first had to elucidate the underlying biomechanical and physiological processes. They knew that the process of metastatic formation occurs in multiple stages. First the metastasizing cells divide from the primary tumor and enter into the vascular system. In the field of cancer research this is referred to as ‘intravasation.’ Then, the cells, also known as circulating tumor cells (CTC), wander through the vascular system. If the CTCs survive this journey, they can leave the vascular system and invade the surrounding tissue. This process, known as extravasation, is completed once the tumor cells embed themselves into the target tissue.
Although there have been many experimental studies on how to describe the various phases of this process, there have not been to date any cell-based computer models capable of simulating the entire process of extravasation of CTCs.
In a recently published article (https://onlinelibrary.wiley.com/doi/epdf/10.1002/cnm.3679), Bäurle, Schneider, and Giehl presented an algorithm capable of doing precisely that. Their computer model is able to predict the temporal evolution of the cell system and changes in the distribution of secreted substances. The researchers found that extravasation of CTCs can actually be broken down into four different phases. Phase I involves ‘chemoattraction,’ whereby CTCs use a chemical substance to attract blood platelets and form CTC-blood-platelet clusters. In Phase II their chemotactic sensitivity is increased and the CTC-blood-platelet clusters are drawn towards the blood vessel wall, induced by a different chemical substance. Phase III involves their penetration into the vasculature wall. Finally, during Phase IV, the wounds in the blood vessel wall are closed and the tumor cells embed themselves in the epithelial cell tissue, a type of protective coating around the blood vessel.
What the model also makes clear: “Even during the final cycles of the simulation, the tumor cells have not yet come to a standstill,” explains Dr. Bäurle. “Their ability to move through the epithelial cell tissue is determined both by the tissue's own movement and through restrictions to their movement imposed by the environment.”
The study shows that their model is in consistency with experimental results and, thus, is capable of simulating the entire process of blood platelet-induced extravasation of CTCs. “This opens the door to the development of new software tools to predict the efficacy of drug-based tumor therapies, and for new experimental work,” says Dr. Bäurle.
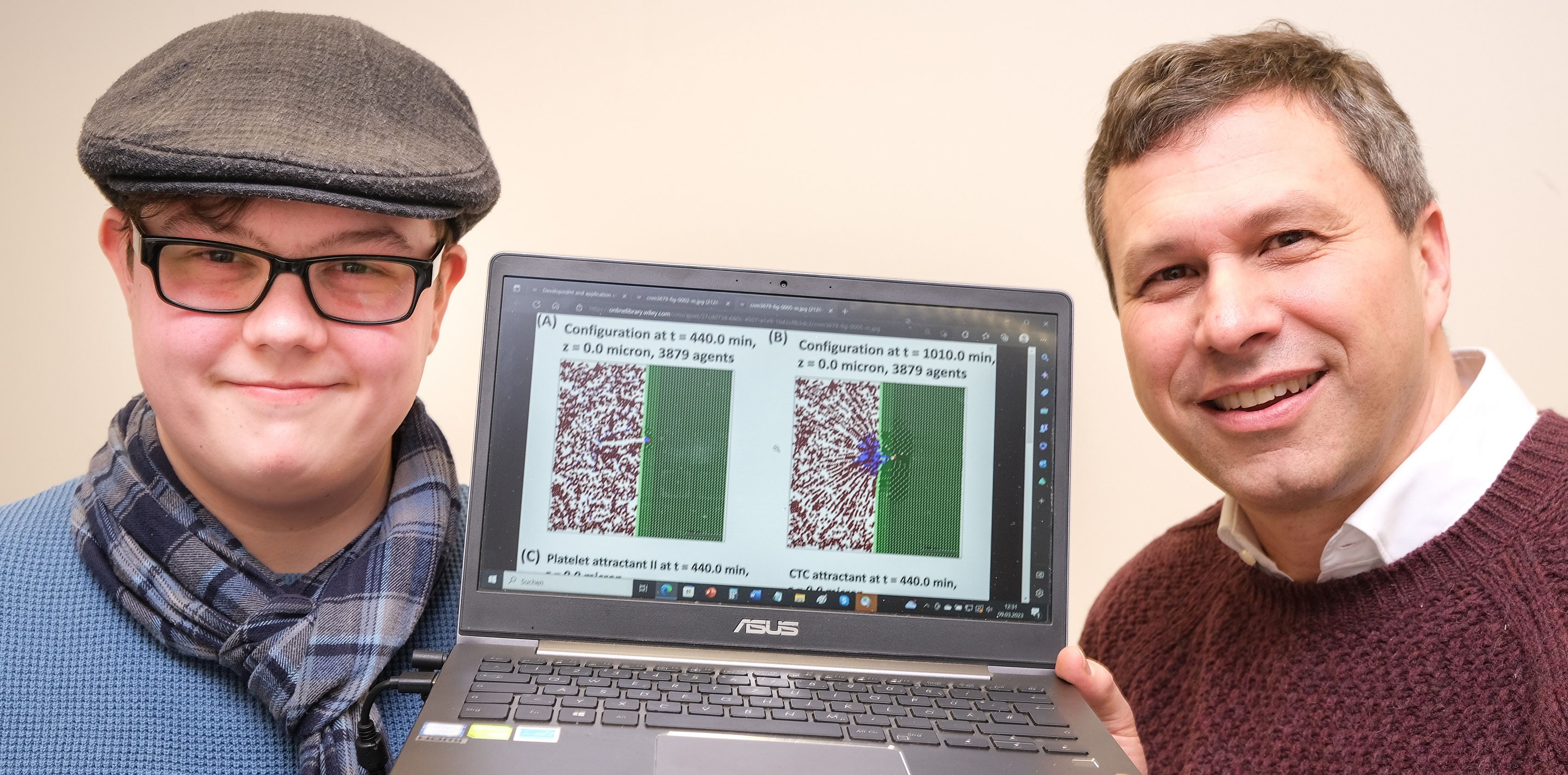
Dr. Stephan Bäurle (right) and doctoral student Kay M. Schneider of the University of Siegen worked together with Cellular Biologist Prof. Klaudia Giehl of the University of Gießen on developing a new computer model for the simulation of metastatic formation.


